ReflowTM
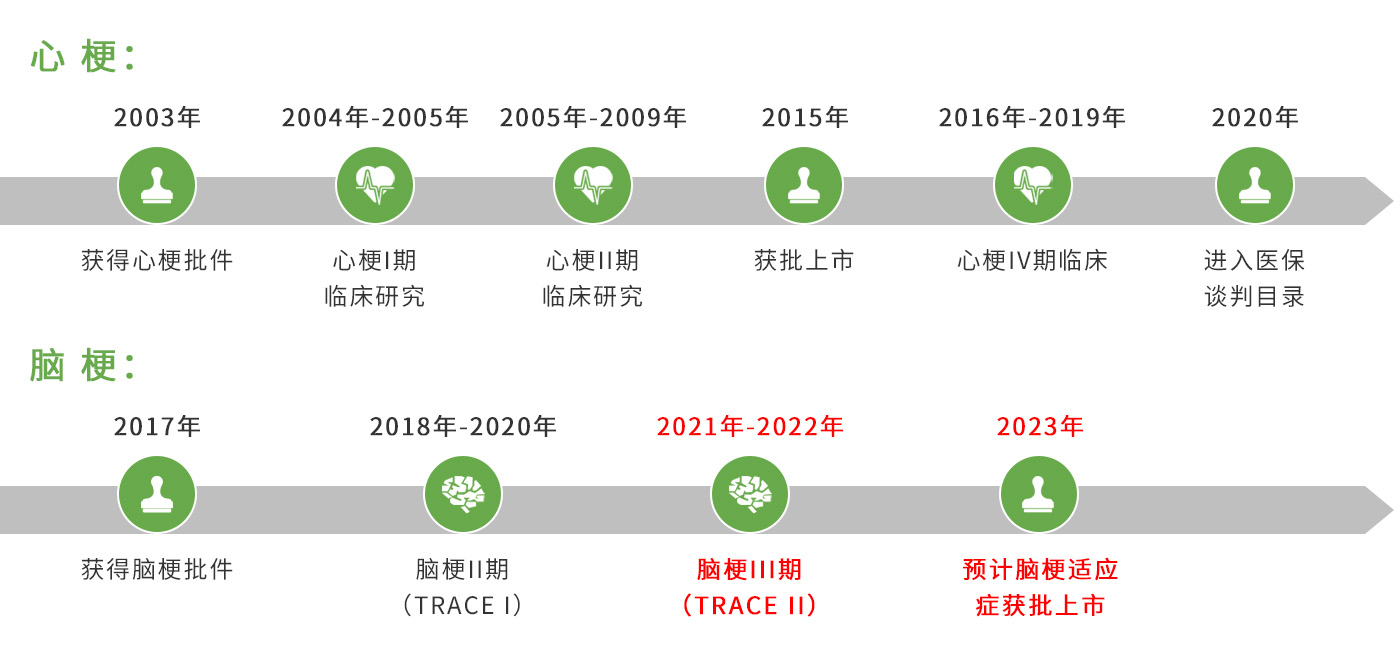
Reflow? (recombinant human TNK tissue-type plasminogen activator, rhTNK-tPA) is a new third-generation specific thrombolytic drug approved in 2015 for the treatment of acute myocardial infarction (AMI). A clinical study of Reflow? on the indication of acute ischemic stroke (AIS) is underway.